Electric Current
Materials conduct electricity because they contain
charged particles that can move around in them.
In solids these charged particles are called
ELECTRONS.
Electrons are tiny negatively charged particles.
In
some materials the electrons can wander about between the atoms, these electrons are called
FREE ELECTRONS.. The more free electrons there are in a
solid the better it will conduct electricity.
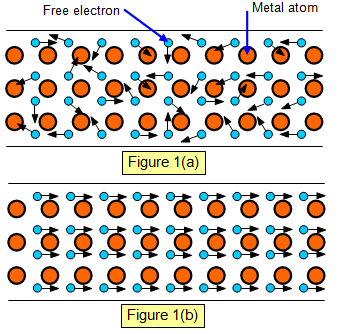
If we now think of the metal as a wire as shown
in the diagram then the tiny electrons show no particular direction of movement (Figure
1(a)).
But if we now connect a battery to the two ends of the wire the electrons drift down the
wire in one direction (Figure 1(b)).
The drift of free electrons in a material is called an electric current.
Materials that will conduct electricity are called electrical
CONDUCTORS. those that won't are called
INSULATORS.

schoolphysics: Electric current animation
To see an animation of the movement of free electrons in an electric current click on the animation link.
Solids that will conduct electricity (by using batteries alone):
all metals
(they contain a lot of free electrons)
carbon
Solids that will not conduct electricity (by
using batteries alone):
dry wood rubber plastic polythene
(they don't contain any, or
many free electrons).
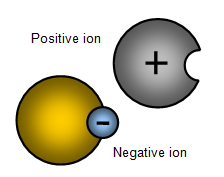
In liquids the current is a flow of CHARGED ATOMS. These charged atoms are called IONS.
Ions come in two types:
POSITIVE
IONS. - atoms that have lost one or more negative electrons
NEGATIVE IONS. - atoms that have gained one or more negative
electrons
The more concentrated a liquid is the more ions there are in it and the better it will
conduct electricity.
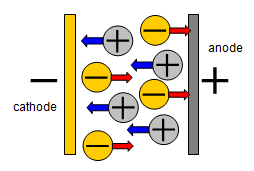
Negative ions will
flow towards the positive electrode in a liquid and positive ions will flow towards the negative
electrode.
Some liquids that will conduct electricity:water (not distilled)
copper sulphate all acids
(they contain a lot of ions)
Some liquids that will not
conduct electricity:paraffin meths distilled water
(they don't contain many or any
ions)
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB