

Diffusion is the movement of one type of gas molecules through
another. Perfume through air, manure through air, food through air, copper sulphate through
water and so on.
Lets think about the diffusion of perfume. The molecules of air in
the room and the molecules of perfume in the spray are all moving around quickly and
randomly (in all directions). The molecules of perfume collide with other molecules of their
own type and also with molecules air. It is very unlikely for any one perfume molecule to be
able to move across the room without hitting another molecule of one type or the other but
they will eventually get there after many collisions. This slow erratic progress is called
diffusion. It is rather like a drunken man staggering across a field through a crowds of excited
soccer supporters. They will eventually make it to the other side but it will take a long
time.
Diffusion can be demonstrated in the laboratory by two or threes simple
experiments.
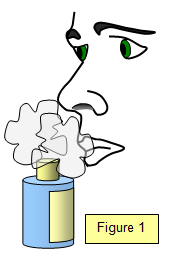
Take a bottle of perfume, remove the stopper and then hold it a few centimetres away from your friend's nose – after a second or two (but not instantly) they will be able to smell the perfume although their nose is not touching the bottle. The perfume molecules have diffused through the air to their nose! (Make sure that they always sniff gently. You never know how unpleasant the smell may be!)
The next two use two dangerous chemicals and so should only be
performed by your teacher.
The first of these experiments is really simple.
Take a bottle of concentrated hydrochloric acid and a bottle of concentrated ammonia.
(Figure 2) Put them one the bench about 20 cm apart and carefully take the stoppers out of
the bottles.
Watch what happens. 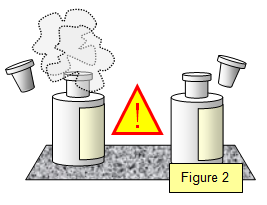
After a
minute or two you should see a white cloud drifting off the top of the hydrochloric acid bottle.
Both the ammonia molecules and the hydrochloric acid molecules have diffused through the
air but the ammonia molecules are lighter and so they will diffuse faster. Where the two types
of molecules meet they react giving the white cloud.

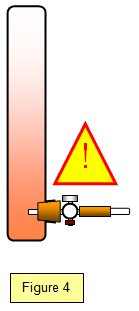
The diffusion of bromine through air is also but since bromine is a hazardous chemical it should only be performed by your teacher in a fume cupboard. A small capsule of bromine is broken in a large glass tube, the brown colour of the bromine gas slowly diffuses through the air. If the experiement is repeated in an evacuated tube the colour reaches the other end in a fraction of the tube a second because there are no air molecules in the way.
