
Using
the apparatus shown in the diagram we can investigate what happens to the pressure of a
gas when it is heated, the volume of the gas being kept constant.
From what you
know about the structure of a gas you should expect that the molecules would move faster
and faster as the gas is heated. Heat energy is being converted into kinetic energy of the
molecules.
This means that they will collide with the walls of the container more
violently and therefore the pressure will rise.
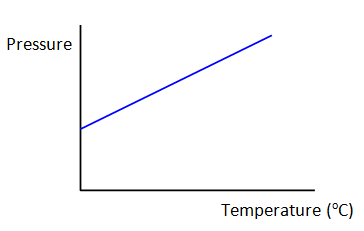
If you plot a graph of pressure against
temperature then you should get a result similar to the one shown in graph 1. It shows that
the pressure increases steadily with increasing temperature.
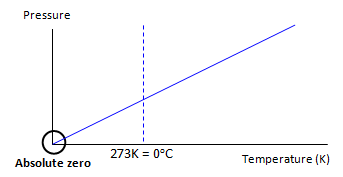
If we draw the line back
to where it cuts the temperature axis (graph 2) we reach a point where the pressure if the
gas is zero – in other words the molecules have stopped moving. They have no velocity and
so no kinetic energy. This is the lowest temperature that it is possible to reach and is called
ABSOLUTE ZERO.
In fact the best definition of
absolute zero is to say that it is the temperature where the gas molecules have their
minimum energy. It is not quite zero energy but very close to it.
On the Celsius scale
absolute zero is therefore -273oC.
 It is useful to use a scale of
temperature that starts at this point and we call this scale the Absolute or Kelvin scale of
temperature.
It is useful to use a scale of
temperature that starts at this point and we call this scale the Absolute or Kelvin scale of
temperature.