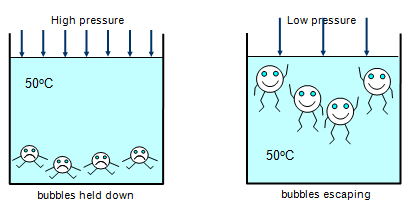

When a liquid boils, bubbles of gas rise from within the body of
the liquid and escape from the surface. This effect occurs at one precise temperature for a
liquid. The pressure of vapour in the bubbles is the same as the air pressure above the surface of the liquid.
If salt is added to water its boiling point goes up because salty water is not the
same liquid as fresh water.
Pressure also affects the boiling point of water. The old
fashioned pressure cooker that was used for cooking worked because the water was being
boiled under high pressure and so its boiling point was raised and this meant that the food in
the water cooked faster. The cooling systems of nuclear reactors also operate under high
pressure enabling water to still be a liquid at over 300oC!
This can be shown by the
following experiment. Set up the apparatus shown in diagram (a) and boil the water. Now
pinch the rubber tube for a few seconds, the pressure in the flask will rise and the
thermometer reading will rise. Don't hold on too long!
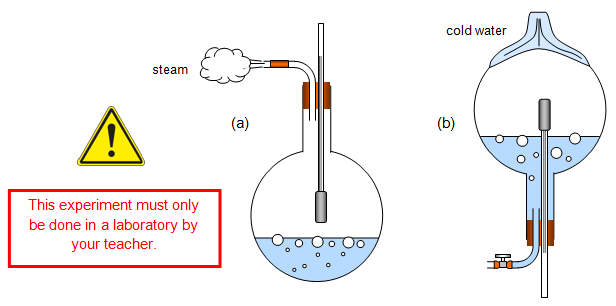
Remove the flask from the heat, clamp the tube and turn it upside down (diagram (b)). Now pour cold water over the flask, some steam will condense in the flask, the pressure will fall and the thermometer reading will go down. You can quite easily make the water boil to below 50oC.