
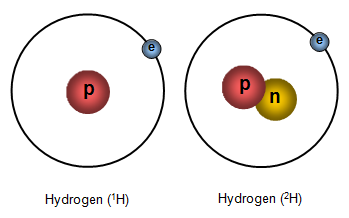
If you look at the pictures you will see that there are two 'versions' of hydrogen. One of them has a nucleus containing a proton but the other has a nucleus with both a proton and a neutron. These two 'versions' of hydrogen are called ISOTOPES of hydrogen.
Almost all elements have naturally occurring isotopes and
many more can be made in the laboratory.
Basically, isotopes of an element are all the
same element (they have the same number of protons) but they have different masses. The
chemical properties of all the isotopes of an element will be the same but their physical properties
will be different because of their different masses. This means that properties like the boiling point
and density of isotopes of an element will be different. For example 'heavy water' containing the
isotope of hydrogen 21H (called deuterium) has a boiling point of 104 oC. Radioactive isotopes (with
different half lives) are used for a variety of purposes – see the section on half-life.
A few
isotopes of some of the elements are shown in the table below.
| Element | Proton number | Neutron number | Nucleon number |
| Hydrogen | 1 | 0 | 1 |
| Deuterium | 1 | 1 | 2 |
| Tritium | 1 | 2 | 3 |
| Carbon | 6 | 6 | 12 |
| Carbon | 6 | 8 | 14 |
| Oxygen | 8 | 8 | 16 |
| Oxygen | 8 | 10 | 18 |
| Neon | 10 | 10 | 20 |
| Neon | 10 | 11 | 21 |
| Neon | 10 | 12 | 22 |
| Cobalt | 27 | 32 | 59 |
| Cobalt | 27 | 33 | 60 |
| Uranium | 92 | 143 | 235 |
| Uranium | 92 | 146 | 238 |
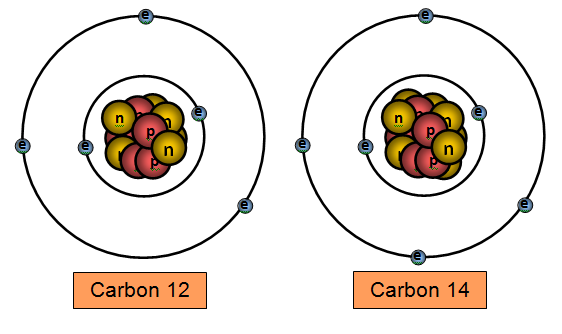
Carbon 14 (6 protons and 8 neutrons) and carbon 12 (6 protons and 6 neutrons) are two
isotopes of carbon.
Radioactive isotopes (radio isotopes) are ones that are
radioactive and emit either alpha, beta or gamma radiation. These are used for a variety of
purposes in medicine and industry.