
All hot
objects emit electromagnetic radiation but the precise frequency and wavelength of that
radiation depend on the temperature of the object.
Let's think about a hot lump of
coal. The coal will emit a wide range of wavelengths – some visible, some ultra violet and
some infrared. At high temperatures there will be a large amount of energy and much of this
will be emitted in the visible part of the spectrum. As the coal cools down there will be less
total energy emitted per second, less visible light and more infrared. When the temperature
has fallen still further the coal will only emit infrared – on a dark night you would not be able
to see it.
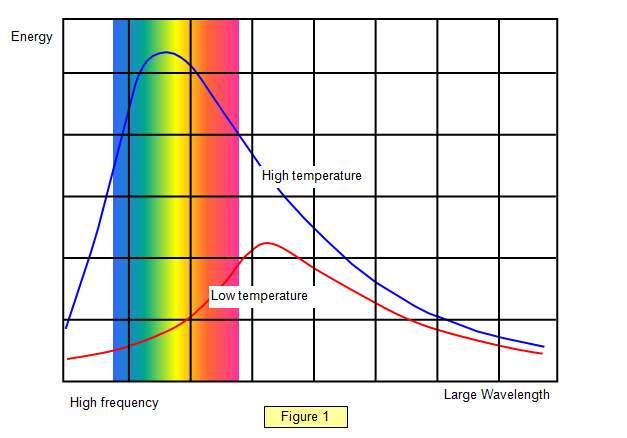
The graph
in Figure 1 shows how the energy emitted per second by a hot object varies with wavelength
and frequency. There are two lines on the graph – one shows an object at high temperature
and the other the same object after it has cooled down.
(Remember that long
wavelength means low frequency. Long wavelength is at the right hand side of the graph and high
frequency at the left hand side).
Notice how the area under the lines changes from when
the object is hot to when it is cool, and also how the position of the wavelength where most
energy is emitted per second moves towards the long wavelength side.
Stars
behave in some ways just like the lump of coal. In the constellation of Orion you can see two
bright examples of hot and cold stars.' The red giant Betelgeuse is a cool star while the blue
giant Rigel, a really hot star, is bluish white. The surface temperature of Rigel is about 10 000
oC while that of Betelgeuse is 'only' about 3400 oC.