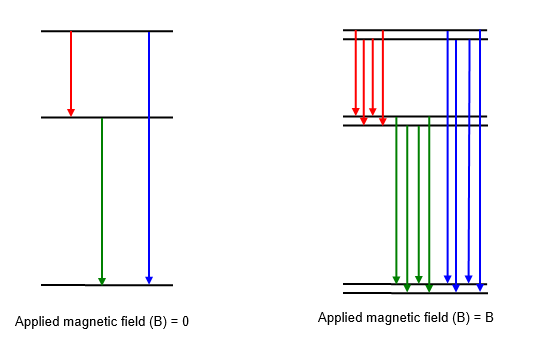

The Zeeman effect concerns the splitting of the lines in a
spectrum. It is the splitting of lines in the radiation emitted by atoms or molecules in a static
magnetic field. Instead of seeing radiation of just one wavelength when an electron transition
occurs between two levels in the atom four lines can be seen since transitions can occur between
the upper and lower levels of the two 'split' energy levels.
It is similar to the Stark effect
which is the splitting of the lines in an electric field.
The splitting the spectral lines due to
the Zeeman effect is directly proportional to the magnetic field applied and so this effect was used
by astronomers to measure the magnetic field of the Sun and other stars.
The Zeeman
effect is named after the Dutch physicist Pieter Zeeman.