
The spectrum of a gas depends on the separation of the electron energy levels within the atom. In an unexcited atom the electron will be in the ground state (level 1) but it can be raised to higher energy levels by the input of energy. This energy may be electrical, radiation, collision with another particle or thermal.
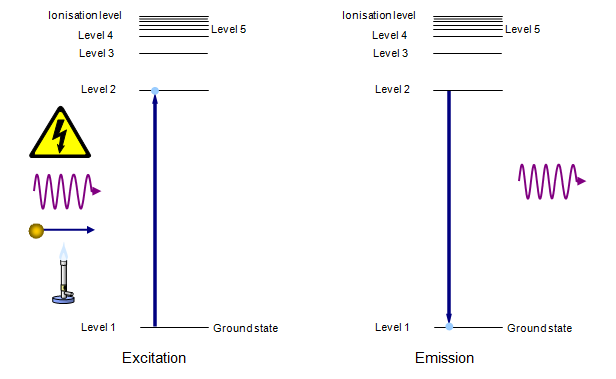
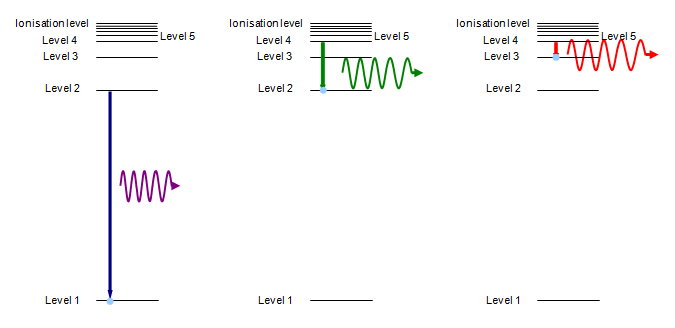
When the electron falls back to a lower energy level (and this usually happens in a fraction of a millisecond) a photon of radiation is emitted. The ‘colour’ of this radiation depends on the energy lost by the electron when falling from the upper to the lower level. The greater the energy drop the shorter the wavelength of the emitted photon – giving blue or even ultraviolet radiation. A schematic version of that is shown below.