
As you
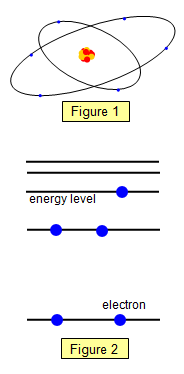
know an atom of an element is composed of a heavy positively charged central nucleus
surrounded by a cloud of orbiting electrons. The size of the positive charge on the nucleus
and the number of orbiting electrons determines the type of element. (See Figure
1).
We can show the state of the electrons in orbit round the nucleus by an energy
level diagram. The energy of each electron is shown on the vertical axis. A simplified version
of an energy level diagram is shown in Figure 2. The electrons are spread through the
energy levels. Notice that no electron can have an energy state between the
levels.
In hydrogen there is just one orbiting electron. The electron is usually in its
unexcited or ground state - level 1 (Figure 3(a).
If energy is put into the atom in the form of radiant energy or by an inelastic collision
with a charged particle (such as another electron) this electron is raised to a higher energy
level and is said to be excited and in an excited state. (In
Figure 3 (b) the electron has been raised to level 3 and the colliding electron has lost some
energy).
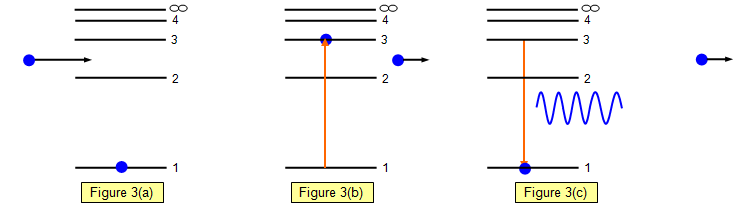
The electron is shown falling
back to its ground state although it does not have to do this. It could fall back to level 2 and
then later on back to level 1.
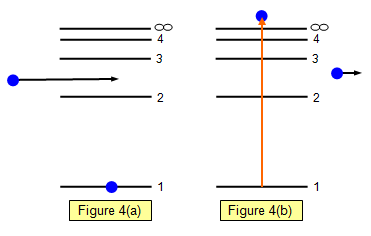
If the collision with an incoming electron is sufficiently
violent an electron within the atom can be given enough energy to raise it to the level marked
with an infinity symbol in the diagrams. (Figure 4 (a and b))
If it reaches this level, or
above, it will escape from the atom altogether. This level is called the ionisation level and the
process is called ionisation.
The removal of one
(or more) electrons will leave the atom with a net positive charge – it has become a positive
ion. To ionise hydrogen requires 21.8x10-19 J or 13.6 eV. This assumes that the
electron starts off in its ground state.