Mass spectrometer
In 1919 Aston developed the first really
good mass spectrograph, an instrument for measuring the masses of isotopes. His
apparatus gave accuracies of one part in 1000.
A simpler form of the mass
spectrograph than Aston's is that due to Bainbridge (1933) and a plan view of this is
shown in Figure 1.
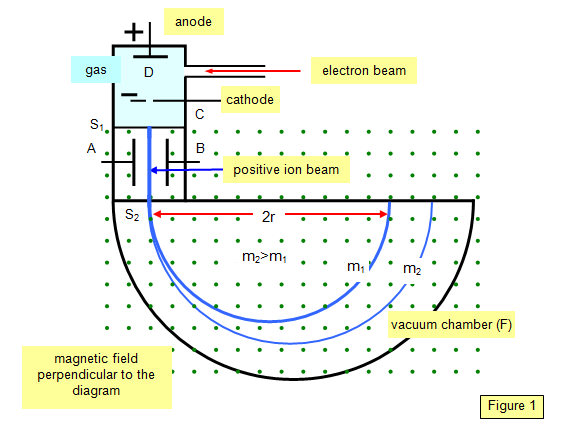
Ions
are formed at D and pass through the cathode C and then through a slit S1. They then
travel between two plates A and B, between which a potential (V) is applied. A magnetic
field (strength B) is applied at right angles to the electrostatic field and so the
electrostatic and electromagnetic forces act in opposite directions to each other.
A
particle with a charge q and velocity v will only pass through the next slit S2 if the
resultant force on it is zero – that is it is traveling in a straight line. That is if:
Electromagnetic force (Bqv) = Electrostatic force (qE)
Therefore, for the
particle to pass through S
2:
Velocity of particle (v) = E/B
But this is a constant, and so only
particles with a certain velocity enter the deflection chamber F. For this reason the
combination of slits and deflecting plates is called a
velocity selector.
In the deflection
chamber the ions are affected by the magnetic fields alone and so move in circular
paths, the lighter ions having the larger path radius. If the mass of an ion is M, its charge
q and its velocity v then:
Bqv = Mv
2/r
where r is the radius of the path.
Therefore r = Mv/(Bq) and so:
Mass of ion (M) = rB2q/E
The radius of the path in the deflection chamber is directly proportional to the mass of the ion.
The detection is by either a photographic
plate or a collector that produces a small current when the ions fall on it. The magnetic
field may be varied, so changing the radii of the particles' paths so that ions of different
masses fall on a fixed collector.
This method of analysis is very accurate and
can detect differences in the masses of two ions as small as one part in 109.
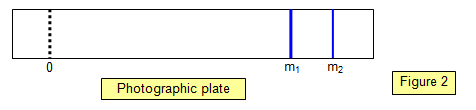
Figure 2
shows the appearance of the photographic plate when a gas containing two isotopes is
used. Note the wider line for the mass m1, showing its relatively greater abundance.
 Student investigation
Student investigation

Figure 3
represents the photographic plate taken from a Bainbridge mass spectrometer. It is
drawn one-quarter full size and shows the collection of two different isotopes. Assuming
that the isotopes are singly charged find
(a) the mass number of each of the
isotopes,
(b) the gas used in the experiment.
The magnetic field across the
apparatus is 0.01 T and the electric field in the velocity selector 100 Vm
-1.

schoolphysics: Mass spectrometer
To see an animation of a mass spectrometer click on the animation link.
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB