Electric Charges, Forces and Fields
Electric
Charge
Static electricity is electric charge at rest, and there are many every day
effects which are due to this. It is well known that if you wear a woollen jumper over a nylon
blouse small sparks can be made when you take it off. A piece of polythene rubbed with a
duster will attract small pieces of paper. A plastic comb rubbed with a cloth will make
someone's hair stand on end if placed near it, a balloon rubbed on a jumper can be made to
stick to the wall and television screens get charged and collect dust on them. Cling film, book
covering film and sellotape can become charged as they are stripped or pulled off the reel.
The mist from a waterfall is charged and a firm making chocolates had to earth the trolleys as
the chocolates had become charged!
Applications of electrostatics:
(a)
electrostatic dust collectors
(b) electrostatic paint sprays
(c) a
photocopier
Electrostatics was probably discovered by Thales in about 600 BC and
to detect static charge we usually investigate the electric field it produces or else discharge
the charged body and observe the current it produces (this is usually small but think about
lightning!) Gilbert (1540-1603) found that he could not charge conducting objects but in 1734
du Fay succeeded in doing this by using an insulating handle. In 1729 Gray found that
charged object could be discharged through the human body to the ground thus giving the
idea of conductors and insulators.
In 1745 du Fay discovered that there were just
two types of static charge, positive and negative, and no more. This was later confirmed by
Benjamin Franklin (1706-1790).
Static charges can be produced by the friction
between two bodies. Two simple sources of positive and negative charge are:
Positive -
glass after being rubbed with silk
Positive - cellulose acetate after being rubbed with a
duster
Negative - polythene after being rubbed with a duster.
In each case the silk
and duster acquires the opposite charge to the solid.
All electric charges exert a
force on each other. This force can be either attractive or repulsive depending on the sign of
the charge.
Two like charges repel each other while two unlike charges attract each other.
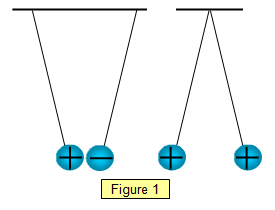
The effect of two charges on each other may be
investigated by hanging up two graphite coated table tennis balls by threads (Figure 1) or by
suspending a polythene rod in a cradle hanging from a fine thread and bringing another
charged rod (of either sign) close to it. Alternatively small pieces of paper can be placed on
the dome of a Van de Graaff generator. When the generator is switched on the paper flies off
– they all have charges of the same sign and the same sign as the dome
itself.
Electric charge is measured in coulombs (C).
An electron has a charge
of -1.6x10-19 C
1 C is the charge on 6.25x1018 electrons!
Remember that this is the same
coulomb mentioned in current electricity where one amp is one coulomb flowing past a point
in the circuit per second.
Electrostatic and gravitational forces - similarities
and differences
We can compare the electrostatic force with that between two
masses (F
G = Gm
1m
2/d
2).
The two forces are similar in that:
(a) they are both inversely proportional to d
2(b) they depend on both the masses or both the
charges
They differ in that:
(a) the gravitational force is always attractive but the
electric force may be repulsive
(b) the gravitational force is independent of the material
placed between the two masses while the electric force depends on the permittivity that
material
Comparison between electric and gravitational forces in a
hydrogen atom
We can now use our knowledge of gravitational and electric force
to find out what is most important in holding atoms together. We will use the example of a
hydrogen atom.
Gravitational force (F
G) = GMm/r
2
Electrostatic force (F
E) =
(1/4πε
o)Q
1Q
2/d
2Now Q
1 = Q
2 since the charge on the electron is equal in size to
the charge on the proton = 1.6x10
-19 C
Mass of a proton = 1.66x10
-27 kg and the mass of
an electron = 9.1x10
-31 kg
So the ratio of the electrostatic force to the gravitational force
within the hydrogen atom
= [(1/4πε
o)Q
1Q
2/r
2]/GMm/r
2 = [(1/4πε
o)Q
1Q
2]/GMm =
9x10
9x(1.6x10
-19)
2/6.67x10
-11x1.66x10
-27 x9.1x10
-31 = 2.3x10
39!
Clearly the electrostatic
force is far more important in this case.
Example problems
1. Calculate the force between two charges - one of 5 C and the other of 25 mC placed 0.06 m apart in
(a) helium.
(b) glycerol
Using:- F = (1/4πε)Q1Q2/d2
(a) εr = 1.00007 for helium
F = 9x109x5x25x10-3/1.00007x(0.062) = 3.12x1011 N
(b) εr = 43 for glycerol
F = 9x109x5x25x10-3/43x(0.062) = 7.26x109 N
2. Two balls of mass 20 mg are each suspended in a vacuum from a non-conducting thread 0.5 m long. If the angle between the threads is 40o calculate the charge on each ball.
Let the tension in each thread be T and the angle between the threads be 2A
and so A = 20o.
Horizontal forces :
Force between the charges = F = (1/4πεo)Q1Q2/d2 = 9x109xQ2/d2 = T sinA
Therefore sinA = 9x109xQ2/Td2
but d/2 = 0.5sinA giving d = sin20 = 0.34
Vertical forces:
T cosA = mg = 20x10-6x9.8 = 1.96x10-4
Therefore: space cosA = 1.96x10-4/T
Therefore: space tanA = (9x109xQ2/0.342)/1.96x10-4 = tan20 = 0.36
so Q2 = 0.36x 1.96x10-4x0.342/9x109
Therefore the charge (Q) on each ball = 30 C
WORD VERSION AVAILABLE ON THE SCHOOLPHYSICS CD