
In 1895 Roentgen was working with discharge tubes when
he discovered that photographic plates placed near the tubes had become fogged although
they had not been exposed to light. He decided that this effect must be due to the emission
of some form of radiation from the discharge tube, and he named the radiation X-
rays.
He deduced that these rays were electromagnetic in nature (it was later
shown that their wavelength was much shorter than that of visible light). He realised that X-
rays were produced when a beam of high energy electrons hits a metal target: the greater
the electron energy, the higher the frequency of the X-rays.
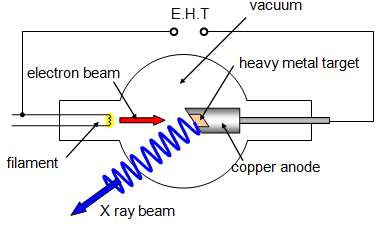
This type of tube
was devised by Coolidge in 1913; it can operate with either a hot or a cold cathode. In the
hot-cathode tube electrons are emitted by thermionic emission and then accelerated by
voltages usually of the order of 20 kV, giving relatively long-wavelength X-rays called 'soft'
X-rays. With a cold cathode, however, the voltages required to cause electron emission are
much greater - around 100 kV - and these tubes produce 'hard' X-rays of much shorter
wavelength, between 10-9 and 10-13 m, depending on the voltages
used. For some applications potential differences of up to 1 000 000 V are
used.
The intensity of the X-ray beam depends on the number of electrons striking
the target per second and in the hot-cathode tubes this is controlled by the heater current.
The wavelength depends on the voltage across the tube. The penetrating power of the X-
rays is thus dependent on the accelerating voltage and the intensity of the beam on the
heater voltage.
When the electrons collide with the target anode they lose their
kinetic energy; some of this energy is converted into X-radiation, but much of it produces
heat. In fact, less than 0.05 per cent of the kinetic energy of the electrons becomes X-ray
energy.
To prevent damage to the anode it has to be cooled, either by air cooling,
using fins, or by pumping cooling liquid through it. It may even be rotated during use to
spread the wear over a larger area.
X-
rays were put to practical use less than three months after Roentgen discovered them. A few
of their present-day applications are as follows:
In art: detecting covered
paintings;
In engineering: checking metal castings for defects; crystal analysis.
In
medicine: there are two main areas
(a) diagnostic. In a simple form, this would be the
detection of a broken bone or a tooth cavity. With the addition of an absorber such as barium
or iodine, X-rays may be used to check respiratory or digestive disorders.
(b) theraputic.
This use is almost completely restricted to the treatment of malignant cancers.
It is
great importance in the use of X-rays for medical purposes that the dose given to both the
patient and the operator is carefully controlled. X-rays can damage living tissue - hence their
use for the destruction of tumours.