Binding energy
One
reason for the stability of a nucleus can be seen if we look more closely at a particular nucleus
such as helium.
Imagine that you were asked to make such a nucleus. You are given the
four pieces (two protons and two neutrons), asked to measure their masses, make them stick
them together somehow and then measure the mass of the finished helium
nucleus.
You will find that the mass of the completed nucleus is different from that of the total mass of the four particles from which it was made! The mass of the nucleus is less than the total mass of the four particles.
This is really quite surprising - it is like taking a cake, weighing it, then cutting up into slices, wieghing them and then finding that the cake had a different mass from the sum of the masses of the slices.
For a nucleus this difference in mass is called the mass defect of the nucleus..
The mass defect for a number of nuclei is shown below.
| 12 Carbon 6 |
0.099 00 |
| 16 Oxygen 8 |
0.137 08 |
| 40 Calcium 20 |
0.367 41 |
| 56 Iron 26 |
0.528 75 |
| 208 Lead 82 |
1.757 84 |
| 235 Uranium 92 |
1.935 38 |
This can best be explained by looking at how easy it
would be to split the alpha particle apart again. If we think of this mass difference as a difference of
energy (using
E = mc2) then the alpha particle has 28.3 MeV less energy than the four
particles. It means that this energy would be needed to split up the helium nucleus. This is called
the
binding energy of a
nucleus.
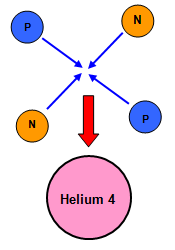
Building an alpha particle
These are the results you would get (all masses in unified atomic mass units (u).)
| protons (2) 2 x 1.007 276 |
= 2.014 552 |
| neutrons (2) 2 x 1.008 665 |
= 2.017 330 |
| Mass of two protons + two neutrons |
= 4.031 882 |
| Mass of "completed" helium nucleus |
= 4.001 508 |
There is a difference of 0.030374 u between them, the helium nucleus is lighter than the four particles that made it!
Example
The mass of the nucleus of the isotope 73Li is 7.016 u.
Find its binding energy.
Mass of a proton = 1.007 273 u Mass of a neutron = 1.008 665 Energy equivalent of 1u = 931 MeV
Protons 3x1.007 273 = 3.021 819 u
Neutrons 4x1.008 665 = 4.034 66 u
Total (3p+4n) = 7.056 479 u
Nucleur mass = 7.016 u
Mass defect = 0.040 479 u
Binding energy = 0.040 479x931 = 37.69 MeV
Further example binding energy problem – working in MeV
Using the data below and that the mass of deuterium (21H) is 2.014102 u calculate the binding energies of 42He and 31H from the following reactions:
21H + 21H gives 32He + 10n + 3.34 MeV
21H + 21H gives 31H + 11H + 4.0 MeV
21H + 31H gives 42He + 10n + 17.6 MeV
Data:
Masses: Proton 1.007 273 u Neutron 1.008 665 u and 1u = 931 MeV
2x1875.129 = 3750.258 gives 32He + 939.067 + 3.34 MeV (1)
2x1875.129 = 3750.258 gives 31H + 937.771 + 4.0 MeV (2)
Therefore from equation (1):
3750.258 – 939.067 – 3.34 = 32He
Mass equivalent of 32He = 2807.851 MeV
Mass equivalent of 2p + 1n = 2814.609 MeV
Binding energy of 32He = 6.758 MeV
Therefore from equation (2):
3750.258 – 937.771 – 4.0 = 31H
Mass equivalent of 31H = 2808.487 MeV
Mass equivalent of 1p + 2n = 2815.905 MeV
Binding energy of 31H = 7.418 MeV
Also: 1875.129 + 31H gives 42He + 939.067 + 17.6 MeV (3)
Therefore: 1875.129 + 2808.487 = 42He + 939.067 + 17.6
Mass equivalent of 42He = 3726.949 MeV
Mass equivalent of 2p + 2n = 3753.682 MeV
Binding energy of 42He = 0.013398 = 26.73 MeV
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB