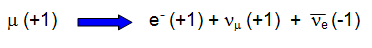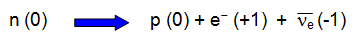

One of the fundamental laws of Physics is that
charge can never be created or destroyed. Charge is always conserved in any reaction. A
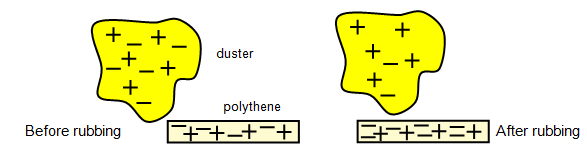
simple example of this is the rubbing of a polythene strip with a duster. Initially the strip and
the dusted were uncharged but after rubbing the strip gains a net negative charge and the
duster gains an equal amount of positive charge – the total charge in the process has been
conserved.
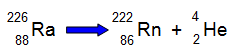
The total
charge on the left hand side of the reaction is zero (88 electrons and 88 protons in the
radium atom). On the right hand side of the equation the total charge is still zero (86 protons
and electrons in the radon atom and 2 protons and 2 electrons within the helium
atom)
The same rules can be applied to the charge at a junction. There is no build
up of charge at a point in an electrical circuit. The charge flowing into the junction is exactly
balanced by the charge flowing out from it.
A further conservation law is that of lepton number. This states that
the lepton number is unchanged throughout a reaction.
Leptons (electrons and
muons with their associated neutrinos) have a lepton number of +1 while antileptons
(positrons, muon plus and their associated antineutrinos) have a lepton number of –
1.
In the decay of a muon (lepton number +1) we have:
Muon (+1) decays
to an electron (+1) + neutrino (+1) + antineutrino (-1)