
All materials
have internal forces that hold the individual molecules together to form a solid. (You should
realise that all these forces are types of the basic electromagnetic force.) The forces in solids
are of four types:
(a) ionic - this is the electrostatic attraction of two
oppositely charged ions and occurs in crystals such as sodium chloride.
(b)
covalent - this force results from electrons being shared between the shells of
adjacent atoms as in diamond, silicon and methane.
(c) metallic - this
force is due to the free electron cloud that exists in metals such as copper. The electrons
move freely between the atoms and are not fixed to any pair of atoms as they are in the
covalent bond.
(d) van der Waals - these are electric dipole forces formed
by the electron cloud and the nucleus; they operate in all matter and are responsible for the
attractive force between molecules in a gas. They can be observed in solid neon, simply
because none of the others operate there.
These forces can give an explanation of
some of the elastic and thermal properties of a material.
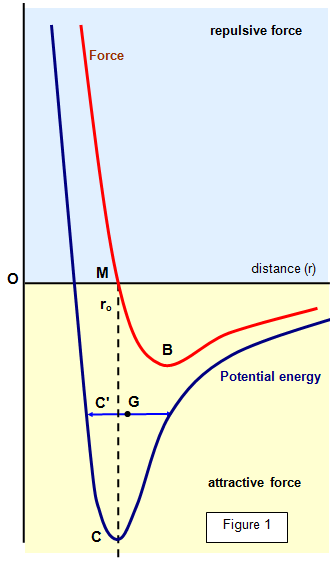
Figure 1 shows how the potential energy of two
molecules and the force between them changes with their separation. The force at any point
is found from F = -dV/dr, where V is the potential energy.
Two forces act between
the molecules:
(a) the repulsive force which predominates at short distances
(b) the
attractive force which predominates at long distances
You can see from the
graph that when the molecules are close to each other the repulsive force predominates,
while at greater distances the attractive force is larger. The resultant force is:
(a)
repulsive from O to M,
(b) attractive from M to B but increasing with distance, and
(c)
attractive from B to infinity but decreasing with distance.
There is a position where
the two forces balance, shown by M on the graph. This is the equilibrium position for
molecules in the solid.
The potential energy is a minimum at this point (as would be
expected). Any disturbance from this position would produce a force tending to return the
molecules to M. The force of attraction between the molecules increases as the molecules
are separated from M to B.
The breaking point is at B, since beyond this point the force of
attraction decreases with increasing separation.
For a molecule to be completely
separated from its neighbour it must gain an amount of energy F, represented by CM on the
diagram. The latent heat of vaporisation for the two molecules is CM when there is no
residual attractive force. This length also represents the latent heat of vaporisation for the
whole material.
In a solid the distance OM is some 2-3x10-10 m and you can see
that around this point the force between the molecules varies approximately linearly with
distance.
The curves also explain the expansion of a solid with increasing
temperature. If an amount of energy F is added to a molecule at C its potential energy will
rise to the level C', the energy appears as kinetic and potential energy and the molecule
oscillates about G. However, since the potential energy - distance curve is not symmetrical
this centre of oscillation is further from O than from M.
This results in a mean
separation of the molecules - that is, an expansion.