
When is a wave not a wave - when it's a
particle!
In 1887 Heinrich Hertz noticed that sparks would jump between two spheres when
their surfaces were illuminated by light from another spark. This effect was studied more carefully in
the following years by Hallwachs and Lenard. They called the effect photoelectric emission and a
very simple experiment can be used to investigate it.
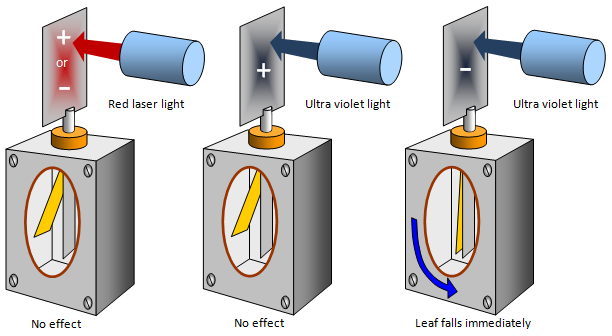
In the diagram shown above a clean zinc plate is fitted
to the top of a gold leaf electroscope and then given a positive charge (you can do this either with a
charged glass rod or an EHT supply. DANGER HIGH
VOLTAGE). The next thing is to shine some radiation on it, using an ordinary lamp, a
helium-neon laser (giving out intense red light) or an ultra violet light has absolutely no effect. The
electroscope stays charged and the leaf stays up.
However if the plate is given a negative
charge to start with (using say a charged polythene rod) there is a difference. Using the lamp and
even the laser has no effect, but when ultra violet light is shone on the plate the leaf falls
immediately, the electroscope has been discharged. (Doing the experiment in a vacuum proves
that it is not ions in the air that are causing the discharge.)
No effect can be produced with
radiation of longer wavelength (lower frequency and smaller energy) no matter how long the
radiation is shone on the plate.
The plate was emitting electrons when the ultra violet
radiation fell on it and this explained why the leaf only fell when it had an initial negative charge -
when it was positive the electrons were attracted back to the plate.
The researchers found
four important facts about the experiment:
(a) no electrons were emitted from the plate if it was
positive
(b) the number of electrons emitted per second depended on the intensity of the
incident radiation
(c) the energy of the electrons depended on the frequency of the incident
radiation
(d) there was a minimum frequency (fo) below which no electrons were emitted no
matter how long radiation fell on the surface
This minimum frequency is called the threshold frequency for that material. Photons with a lower frequency
will never cause electron emission. This can be explained like this.
The free electrons are held
in the metal in a "hole" in the electric field, this is called a potential well. Energy has to be supplied
to them to enable them to escape from the surface. Think of a person down a hole with very
smooth sides. They can only escape if they can jump out of the hole in one go. They cannot get half
way up and then have a rest - it's all or nothing!. This is just like the electrons. The deeper the
"hole" the more tightly bound are the electrons and the greater energy and therefore greater
frequency of radiation is needed to release them.
The quantum theory of Max Planck is
needed to explain the photoelectric effect. In trying to explain the variation of energy with
wavelength for the radiation emitted by hot objects he came to the conclusion that all radiation is
emitted in quanta and the energy of one quantum is given by
the equation:
| Element | W (Joules) | W (eV)(V) | fo (frequency) (Hz) | λo (wavelength) (nm) |
| Sodium | 3.8x10-19 | 2.40 | 5.8x1014 | 520 |
| Caesium | 3.0x10-19 | 1.88 | 4.5x1014 | 666 |
| Lithium | 3.7x10-19 | 1.88 | 5.6x1014 | 560 |
| Calcium | 4.3x10-19 | 2.69 | 6.5x1014 | 462 |
| Magnesium | 4.3x10-19 | 3.69 | 8.9x1014 | 337 |
| Silver | 7.6x10-19 | 4.75 | 11.14x1014 | 263 |
| Platinum | 10.0x10-19 | 6.75 | 15.1x1014 | 199 |
Another way of looking
at it is to think of a fairground coconut shy. A brother and sister are trying to knock the coconuts off
their stands. The boy has a large box of table tennis balls which he is throwing at the coconuts, with
little effect. No matter how many of the table tennis balls he throws at a coconut it will still stay in
place – the table tennis balls represent the "red" quanta. However his sister has a pistol! This
represents the violet quanta. A single shot from the pistol will knock off a coconut and it will do it
immediately.
As we saw in the previous experiment we could illuminate the
zinc plate all day with a high powered laser and the leaf of the electroscope would not fall. However
as soon as we shone the ultra violet light on the plate the leaf dropped. This is because the ultra
violet light has a high enough frequency and therefore each quantum of ultra violet has sufficient
energy. One quantum has enough energy to kick out an electron in one go.
The
photoelectric effect is therefore very good evidence for the particulate nature of light.