

A copper or aluminium calorimeter, a muff, an electrical immersion heater, a voltmeter, an ammeter, connecting leads, a low voltage power supply, a thermometer (0 – 50oC), a stop watch.
Measure the mass of the calorimeter (MC) and fill it with a known mass of water (MW).
Record the initial water temperature (θ1).
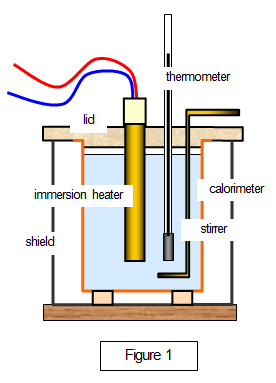
Set up the apparatus as shown in Figure 1.
Switch on the heater allow it to heat up so that it is slightly warm to the touch and then put it in the water. Place the muff over the calorimeter and heat the water for a measured time (t).
Measure the final temperature of the water (θ2).
Record the voltage (V) and current (I), this may need to be adjusted throughout the experiment so that the power input remains constant.