
We have considered the forces that exist in a solid between two adjacent
molecules and we have therefore assumed the existence of these molecules.
The follow-
ing simple experiment can be used to give a rough idea of the size of a molecule, and hence an
atom.
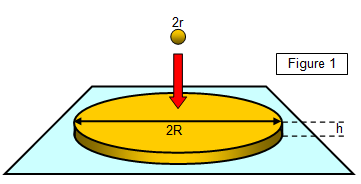
The radius r of a small drop of oil is found and the volume of the drop
calculated. The drop is now placed on the surface of some dust-covered water and the drop
spreads out into a roughly circular patch of height h and radius R. The diameter of the patch is
measured and hence the radius R is found.
Now the volume of the original drop (4/3πr3) is the
same as that of the film (πr2h); therefore, if h is the thickness of the film and the original volume of the
drop is known the thickness of the film can be found.
Now the thickness of the oil film
cannot be less than the size of a molecule and so the size of a molecule of oil must be equal to or
less than the thickness of the film.
If the thickness of the film (h) can be found this is the upper
limit of the size of an oil molecule.