
All bodies at all temperatures emit
radiation, the intensity and wavelength distribution depending on the nature of the body itself and
its temperature. At temperatures below about 500 oC (773 K) the radiation emitted is in the
Infrared region of the electromagnetic spectrum. This radiation is invisible to the human eye but its
detection is much used in Earth resource satellites, in remote control devices for televisions and
stereos, by the military in night glasses, in heat seeking missiles, by the rescue services in locating
buried people in collapsed buildings, by the fire service for "seeing" in smoke filed rooms and for
detecting heat loses from buildings and power cables.
Snakes have infra red detectors below their
eyes so that they can detect the heat emitted by their potential prey! Reflecting capes and the
silvering on a vacuum flask exploit the low emissive properties of shiny surfaces. Infrared radiation
was first detected by Herschel in 1820, when he showed that there was radiation beyond the red
end of the visible spectrum.
Infrared
radiation can be shown to be electromagnetic in nature and to have a wavelength rather longer
than that of visible light. In fact the infrared region of the spectrum extends from about 750 nm to
some 400 000 nm (400 mm, 0.4 mm).
We can demonstrate
the reflection of infrared radiation quite easily because the surfaces do not need to be very flat to
give good reflection. Refraction is more difficult to show because many materials are opaque to
infrared. Glass is one of these, only short wave radiation passing through. Glass will actually
transmit up to 3000 nm, fluorite up to 9000 nm and rock salt up to 15 000 nm so clearly lenses and
prism designed to refract infrared should be made of rock salt.
An infrared filter that will
transmit infrared but be completely opaque to visible light can be made from a solution of iodine in
carbon disulphide. The velocity of infrared can be inferred from a solar eclipse; infrared radiation
and light are cut off at the same instant. This simple observation suggests that in free space the
velocity of infrared radiation is the same as that of light.
Infrared
radiation
All bodies emit radiation, the intensity and wavelength
distribution depending on the nature of the body itself and its
temperature. Infrared radiation is invisible to the human eye. The
detection of infrared radiation is much used, however, in Earth resource
satellites, by the military in night glasses, for spotting areas of high
heat loss from buildings and by the electricity boards in detecting hot
spots in power cables.
It had been thought that only 'hot' bodies emitted
radiation, but of course what may be 'hot' when compared with one set of
surroundings may be 'cold' when compared with another. For example, a
candle flame (700 oC) seems hot compared with your hand (37
oC) but cold when compared with the surface of the Sun (6000
oC).
In 1792. Prévost suggested that all bodies radiate
energy, but that those with a higher temperature radiate more energy
than those at lower temperatures.
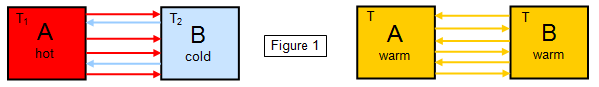
If we consider two isolated
bodies A and B initially at different temperatures (T1 and
T2) with A being hotter than B (Figure 1), then each body will
radiate heat to the other. The result will be equal temperatures (T), a
kind of 'smearing out' of heat energy over the whole system.
Notice
that it is finally a case of dynamic equilibrium, both bodies radiating
an equal amount of energy to each other.
The
amount of infrared radiation emitted by a body depends on three things:
(a) the surface area of
the body
(b) the type of surface
(c) the temperature of the body
Consider first the
type of surface. Simple experiments like those with Leslie's cube show that rough black surfaces
makes the best emitters and absorbers of radiation at a given temperature.
An ideal absorber
would be one that absorbed all the radiation that fell on it and also emitted the maximum amount
of radiation possible for that area at that temperature. Such a body is known as a black body and
the radiation emitted by it as black body radiation.
It is important to realise the difference
between this equation and Newton's law of cooling. Stefan's law applies to the loss of energy by
radiation while Newton's law applies to loss of energy by convection. Both laws are found to hold
for temperature differences of hundreds of degrees.
Satellites – passing from bright sunlight into the earth's shadow. The
change in the incident radiation would cause expansion and contraction of the satellite and so it
must be reflective coating to prevent excessive heating/cooling .
Bedouin wear black clothes -
absorb heat and then give good thermal convection currents between layers of
clothing.
Reflective capes are given to the runners at the end of a marathon to prevent/reduce
heat loss from the body by radiation