
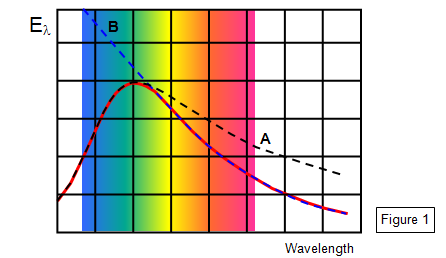
When scientists were attempting to deduce equations that would fit the black body radiation curves they encountered considerable difficulty - none of the equations that they produced using classical Physics would fit the experimental results (shown as the red line). The curves either fitted well for the ultra violet (line A) but were unsatisfactory at longer wavelengths or they were fine for infrared but suggested far too much energy in the short wavelength region – the so- called ultra violet catastrophe (line B). (See Figure 1)
However in 1900 Max Planck solved the problem by proposing a radical new idea to govern the emission and absorption of radiation. He suggested that the energy was not radiated in a continuous wave but in discrete packets which he called quanta and that the energy (E) of each quantum was given by the equation:
where f is the frequency
of the radiation and h is a constant now known as Planck's constant.
(For a fuller
treatment of the quantum theory see 16-19/Quantum physics/Text/Quantum theory of
radiation)
Furthermore each quantum could only have certain energy values, but no
value between these. This fact would not be obvious to use in our large-scale world but is
something that becomes vitally important at an atomic level.
It was therefore here in the
study of heat radiation and not in the realms of nuclear physics that the quantum theory was
born.
Planck deduced an equation for the energy distribution of black body
radiation based on his quantum theory that fitted the experimental results
exactly.
| Eλ | the energy density | |
| λ | the wavelength | |
| T | the absolute temperature of the body | |
| h | Planck's constant | |
| c | the speed of electromagnetic radiation in free space | |
| a | a constant equal to ch/k where k is Boltzmann's constant (a = 1.44x10-2 mK) |