
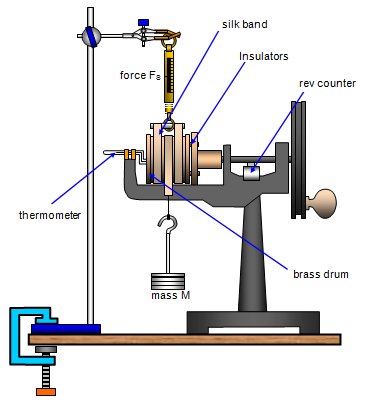
The method described below was proposed by Joule in 1847 and the apparatus shown in the diagram was devised by Callendar. This was originally intended to measure the mechanical equivalent of heat.
This was the mechanical
energy needed to produce a known amount of heat energy.
A silk band is
wrapped round a brass drum (mass mB and specific heat capacity cB) with
one end attached to a spring balance (S) and the other to a weight (Mg) that hangs
freely. A small amount of water (mass mW) is put in the drum and the
temperature of the water is measured using a bent thermometer.
The drum
is rotated by hand using the wheel at the right and the spring balance reading
(FS) recorded.
The frictional force between the band and the drum
is:
F = Mg – FS
The couple
exerted on the drum by this force is Fr where r is the radius of the drum.
If
the drum makes N revolutions the work done is 2πNFr
