
If a nucleus
is made unstable it may lose the extra energy in two ways. It can emit radiation (alpha, beta or
gamma) or undergo fission. You can think of fission as rather like a wobbly jelly that has been
shaken about too much and simply split up.
In all nuclei there are two forces, the strong
nuclear force (acting between the neutrons and protons) trying to hold the nucleus together
and the electrostatic repulsion trying to push the protons apart.
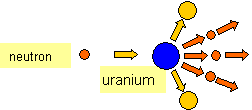
When a neutron is fired at a uranium 235 nucleus the neutron is captured and uranium 236 is
formed. This is an unstable nucleus and it splits into two. This is known as nuclear fission.
| 235U + 1n | capture | 236U | fission | 148La + 85Br + 31n + energy |
| 235.044 + 1.0087 | 147.961 + 84.938 + 3.0261 |
This reaction has a mass defect of 0.1276u
Energy is given out by
the reaction because the mass of the products is less than the total mass of the original
nucleus and the neutron.
A full treatment of the equation shows that this energy is
118 MeV or 1.90 x 10-11J. This is a very small amount of energy but when you work out how
many nuclei there are in 1kg of uranium you can understand why nuclear fission is so
important.