Coulomb's Law of force between charges
The force between two
charges Q
1 and Q
2 separated by a distance d is expressed by
Coulombs law, proposed in 1785:
Coulomb's law for the force (F) between two charges: F = (1/4πε)Q1Q2/d2
where ε is a constant for a given material known as
the
permittivity of that material. Basically changing
e changes the force between the two charges, for example if
e is large the force is small.
In a vacuum this equation
becomes
Coulomb's law for the force (F) between two charges in a vacuum: F = (1/4πεo)Q1Q2/d2
where ε
o is the
permittivity of free space (8.84x10
-12 Fm
-1)
A useful quantity in
calculations is 1/4πε
o - this is equal to 9x10
9 F m
-1
Notice that this force is:
(a) proportional to 1/d
2(b) dependent
on the electrical properties of the material between the charges (ε)
(c) it may be attractive
or repulsive
It is often useful to compare the electrostatic properties of material by using
a quantity known as the relative permittivity (ε
r). This is defined using the relation:
ε = εoer
Some examples of relative
permittivities (at 293 K) are given in the following table:
| Solid |
|
|
Liquid |
|
|
Gas |
|
| Amber |
2.8 |
|
Castor oil |
4.5 |
|
Air |
1.000 0536 |
| Ebonite |
2.8 |
|
Glycerol |
43 |
|
Carbon dioxide |
1.000 986 |
| Glass |
5-10 |
|
Ethoxyethane |
4.3 |
|
Helium |
1.000 07 |
| Mica |
5.7-6.7 |
|
Nitrobenzene |
35.7 |
|
Hydrogen |
1.000 27 |
| PVC |
4.5 |
|
Paraffin |
2.2 |
|
Nitrogen |
1.000 580 |
Polystyrene |
2.55 |
|
Propanone |
21.3 |
|
Oxygen |
1.000 53 |
| Teflon |
2.1 |
|
Turpentine |
2.23 |
|
Water vapour (393 K) |
1.000 60 |
| Wax |
5-10 |
|
Water |
80.4 |
|
|
|
The
force between two point charges and between two charged plates
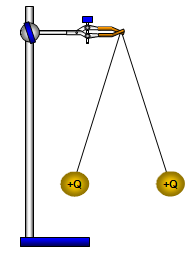
(a) two point charges
this can be done
with two light balls suspended on threads. They are given charges of equal sign and repel
each other. By measuring the angle between the threads and the masses of the balls the
force between them can be found and the variation of force with charge can be
investigated.
It is interesting to discuss what would happen if the masses of the two balls
were the same but the charges on them were different. Would the threads make the same
angle with the vertical?
(b) two parallel plates
The
formula for the force between two plates is F = ½ [QE] = ½ [QV/t] and using the relation for
the capacitance of a parallel plate capacitor of area A and separation t (C = Q/V = εoA/t) the
force between two charged plates is:
F = ½[ εoAV2/t2]
It is very simple to test this using a top pan balance and
two aluminium plates.
Example problems
1. Calculate the force between two charges of 10-5 C separated by a distance of 0.2 m in a vacuum.
Force (F) = [1/4πεo][Q2/d2] = [9x109x10-10]/0.22 = 2.5 N
2. Calculate the value of two similar charges separated by 0.5 m in a vacuum if the force between them is 1.5 N.
Q2 = [4πεo][Fd2] = [1/9x109]x[1.5x0.25] = 4.17x10-11
Q = 6.45x10-6 C
Electrostatic and gravitational attraction
It is interesting to compare the electrostatic and gravitational attraction betwen two charged masses, for example an electron orbiting and hydrogen nucleus.
The gravitational attraction between them is 4x10
-47N while the electrostation attraction is 8x10
-8N, about 10
39 times stronger.