
The molecules in a liquid are in a state of continuous motion and some of
those at the liquid surface will gain sufficient energy to escape from the surface altogether.
The molecules that have left the surface are said to be in the vapour state. The difference
between a vapour and a gas is purely one of temperature, a vapour being a gas below its
critical temperature.
This phenomenon is known as evaporation. The number of
molecules leaving the surface, and hence the rate of evaporation, will increase with
temperature as the liquid contains more energy at a higher temperature. The effect of the
evaporation of a liquid can be shown clearly by the following experiment.
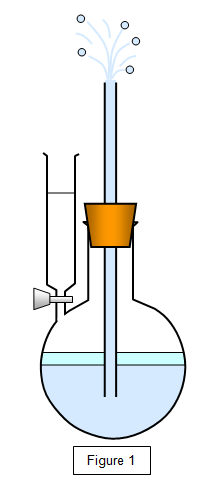
Some
ether is run into the flask, as shown in Figure 1. It will evaporate in the enclosed space and
the pressure that it exerts on the water will force a jet of water out of the tube. Warming the
liquid will increase this evaporation and give a more powerful jet.
You can show that
the rate of evaporation may be increased by:

(a) warming the flask gently,
(b)
increasing the area of the liquid surface,
(c) blowing a stream of air across
the surface, and
(d) reducing the pressure above the liquid surface.
When a liquid is in a closed
container the space above the liquid is full of vapour, and the vapour is then described as a
saturated vapour - this means that the density of the liquid molecules in the air is a
maximum. This is due to molecules continually escaping and reentering the liquid. At any
moment the number of molecules leaving the surface will be equal to the number returning
to it and so a dynamic equilibrium is set up.
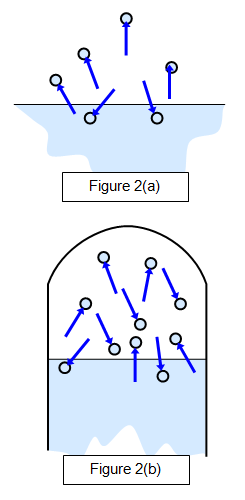
The properties of saturated vapours
were first investigated by Dalton around 1800. This is shown in Figure 2(a), which shows a
state before saturation has been reached (when there will be more molecules leaving the
surface than returning to it) and Figure 2(b), which shows the saturated state. A dynamic
equilibrium exits here.
This vapour will exert a pressure and if there is sufficient
liquid the air above the liquid surface will be saturated with vapour; the pressure that this
saturated vapour exerts is known as the saturated vapour pressure (s.v.p.) of the liquid at
that temperature.
Notice that since the velocity of the molecules
increases with temperature the saturated vapour pressure also increases with temperature,
and therefore the temperature of the vapour must be specified when quoting its saturated vapour pressure (s.v.p.)
| Temperature (oC) | S.V.P (MPa) | Temperature (oC) | S.V.P (MPa) | |
| 10 | 0.001 227 | 50 | 0.012 34 | |
| 15 | 0.001 704 | 60 | 0.019 92 | |
| 20 | 0.002 337 | 70 | 0.031 16 | |
| 25 | 0.003 166 | 80 | 0.04736 | |
| 30 | 0.004 242 | 90 | 0.070 11 | |
| 40 | 0.007 375 | 100 | 0.101 325 |