The pressure of the atmosphere
To inflate a balloon by stretching the rubber skin you must blow air into it.
As a high altitude balloon goes up it gets bigger.
If you want a comfortable ride in a car or on a bike you must pump up the tyres.
It is very difficult to pour liquid out of a tin if only one small hole is made in the tin but much easier if another hole is made in the tin on the opposite side of the lid.
When you put a straw into a glass of lemonade and suck, the lemonade goes into your mouth.
When you press the button on an aerosol can a spray comes out.
We live at the bottom of an ocean of air – we call it the
ATMOSPHERE. It presses on us from all directions, and
it's only the pressure of our blood and the air in our bodies that stops parts of us from being
squashed. If we think of the air in the atmosphere to be like a liquid then our ocean of air
would be about 8000 m deep! Obviously if we lived at the bottom of an ocean of 8000 m of
water the pressure would be enormous so the density of air must be much lower than that of
water.
The pressure of the air at sea level is about 100 000 N/m2 (Pa).
This is like having two quite heavy people standing on your head.
You can show the
effects of air pressure either by trying to move something through the air or by looking at the
effect of air pressure on an object containing no air.
Effects of air pressure
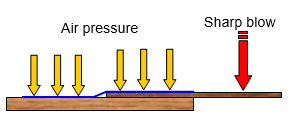
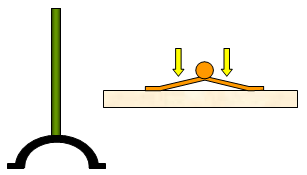
If you
put a piece of thin wood on the desk with the end sticking over the edge, spread some
newspaper over the wood on the desk and then give the protruding piece of wood a sharp
blow with your hand, the wood will break. This happens because the pressure of the air on
the paper stops it from lifting.
Using a blowpipe you can melt a small hole in a light
bulb or an old wireless valve. There is almost no air inside and so the air outside tries to get
in. As the glass melts, the air pressure pushes it inwards.
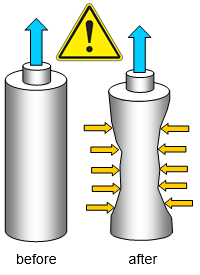
If you remove the air from a tin can there is
nothing inside to hold it up and so you can see the effects of air pressure on it.
You
can take the air out with a vacuum pump or boil a little water in it (with the lid off) so that the
steam will drive out the air. lf you do it the second way the steam inside the can will turn
back to water when you stop heating. A vacuum will be left above the water if the cap is
replaced upon removing the can from the heat. The pressure of the air on the outside will
then be enough to squash the tin!
It is the difference in pressure
between the inside and outside of the tin that crushes it.
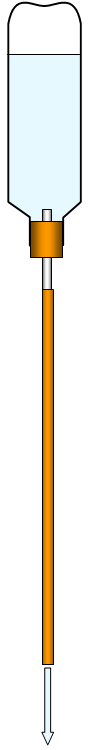
An alternative demonstration is to use a plastic
drinks bottle. Fill it with water, fix a long tube to it and then simply hold it upside down. The
water runs out leaving a vacuum and the pressure of the atmosphere outside the bottle
squashes it.

Two other
interesting effects of air pressure are shown in the next diagram:
(a) shows a rubber
sucker
(b) a glass full of water — upside down.
(a) When you push a rubber
sucker on to a surface you force all, or nearly all, the air out of it leaving a partial vacuum
inside. The much greater pressure of the air outside will hold the sucker to the surface.
A rubber sucker on the end of a handle can be used to help unblock a drain leading from
a sink. Can you explain why?
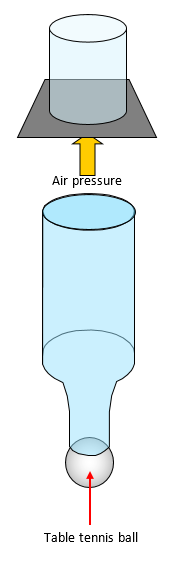
(b) If a glass is filled with water and a card put over
the top you can turn the glass upside down without the card coming off. This is because the
water pressure above the card is less than the pressure below the card. What is the largest
glass that this would work with? An alternative method is to use a milk bottle and a table
tennis ball.
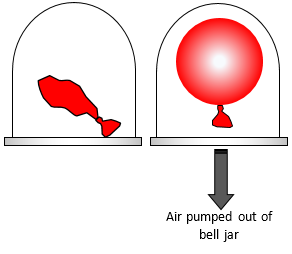
If you put a partly blown up balloon in a bell jar and then pump out the
air from the bell jar the balloon will slowly expand. This is because the air inside the balloon
is at a fairly high pressure and when the air outside the balloon is removed there is a bigger
pressure difference between the inside and outside of the balloon. The balloon therefore
expands to balance this difference.
The
Bourdon gauge
The Bourdon gauge is an instrument that measures gas
pressure. It works in the same way as the simple paper blowers that you use at parties.
When gas is forced into the closed metal tube of the Bourdon gauge it uncoils in just the
same way as the party blower does when you blow into it. This small uncoiling is measured
by a pointer and a scale.
Problems
1. Explain how rubber suckers could be used to help lift panes of glass safely.
2. If you wanted to hang from the ceiling using a rubber sucker how big would it have to be?
(Assume a perfect vacuum in the sucker. Take atmospheric pressure to be 100 000 Pa and use your own weight in Newtons).
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB