

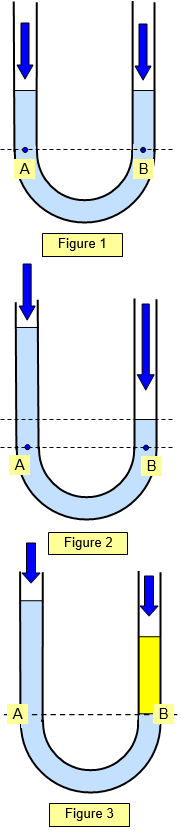
In Figure 2 the U tube
is still partly filled with liquid but this time someone blows into the right hand open end. The
pressure at the points A and B must still be the same (same level in the liquid) and so the
height of the column of liquid on the right is less than that on the left.
The pressure at A is
due to the ordinary air pressure plus the height of the column above A and the pressure at B
is the higher air pressure plus the liquid column above B.
In Figure 3
the U tube contains two different liquids. The yellow liquid on the right has a higher density
than the blue liquid on the left and so less of it is needed to give the same pressure at points
A and B. The pressure at the points A and B must still be the same.
The pressure at A is
due to the ordinary air pressure plus the height of the column above A and the pressure at B
is the higher air pressure plus the liquid column above B.
If the pressure on one limb of a U tube
is greater than the other this difference in pressure can be measured by simply finding the
DIFFERENCE in height (h) of the liquid in the two limbs of the U tube. In the diagram shown
here (Figure 4) this difference is 11 cm.
This apparatus can be used to measure the
pressure of your lungs above atmospheric pressure by simply blowing into one end of a long
U tube and the finding the difference in liquid levels. If water is used as the liquid in the U
tube your lung pressure will be anything between about 50 and 200 cm of water. This means
that you could hold up a column of water between 50 and 200 cm high just by
blowing.
The actual pressure above atmospheric in pascals can be
worked out using the formula:
Pressure = depth x density x gravity = 0.11 x 1000 x
10 = 110 Pa
(Acceleration due to the Earth's gravity = 10 m/s2 and density of
water = 1000 kg/m3)