Conversion of electrical energy to heat energy
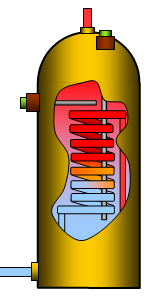
Many homes have immersion heaters for heating the water and the two
examples given here show how the heating effects of immersion heaters can be calculated.
The first example uses a 50 W heater, you have probably used one in school and the second
is a 3 kW immersion heater heating water for a bath.
Assuming that the all the electrical energy produced is converted to heat
energy we can write:
Electrical energy input = Heat energy absorbed by the object
But:
Heat energy = mass x specific heat capacity x
temperature change and
Electrical energy = Volts x Amps x time = Electrical Power x
time
Therefore:
Heat energy = mass x specific heat capacity x temperature rise = VIt = Power x time
Example problem
A 50 W heater is used to heat a 2 kg block of aluminium. If the temperature rise after 10 minutes heating is 15oC what is the specific heat capacity of aluminium?
Heat energy input = 50x10x60 = 30 000J
Heat energy gained by aluminium = 2 x specific heat capacity x 15 = 30 000J
Specific heat capacity of aluminium = 30 000/30 =1000J/(kgoC)
Notice that the time (10 minutes) has been converted into seconds.
In
this example it has been assumed that all the heat given out by the heater has been
absorbed by the aluminium, in fact some will be lost to the air.
Example problem
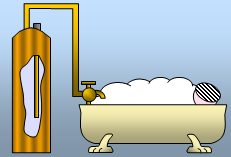
A 3kW immersion heater is used to heat 80kg of water for a hot bath. The water starts at 18
oC and is heated to 43
oC - a rise of 25
oC. How long will this take?
Mass of water needed = 80kg
Rise in temp needed = 25
oC
Power of heater = 3 kW = 3000W
Specific heat capacity of water = 4200 J/(kg
oC)
Heat energy required = 80 x 4200 x 25 = 8400 000 J
Time for heater to produce this amount of energy
= 8400000/3000 = 2800 s = 46.7 minutes
A VERSION IN WORD IS AVAILABLE ON THE SCHOOLPHYSICS USB


 A 3kW immersion heater is used to heat 80kg of water for a hot bath. The water starts at 18oC and is heated to 43oC - a rise of 25oC. How long will this take?
A 3kW immersion heater is used to heat 80kg of water for a hot bath. The water starts at 18oC and is heated to 43oC - a rise of 25oC. How long will this take?