
If
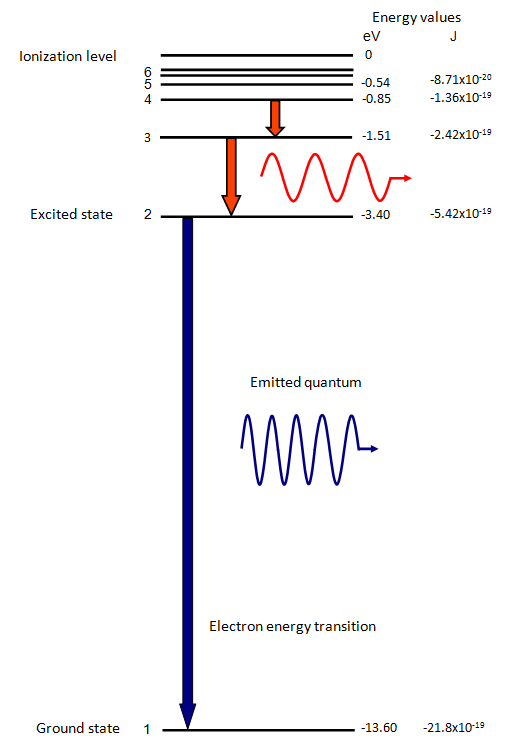
an electron from a low level is given energy it will be raised to a higher, or excited, level. This can
be done electrically, by heat, by collision with another atom, by radiation or by a free electron hitting
the atom. However the electron in the atom will only be excited if the energy of the incoming
quantum of energy in whatever form is exactly the same as the difference in energy between the
two levels.
When an electron falls from one level to another energy is emitted in the form
of a quantum of radiation. The energy of this quantum and therefore its frequency and wavelength
is determined by the difference in energy between the two levels.

| Level | Energy (eV) | Energy x10-19(J) | Transition | Energy (ev) | Frequency(Hz) x1014 | Wavelength (nm) | |
| ∞ | 0 | 0 | ∞ to 1 | 13.6 | 32.9 | 91 | |
| 6 | -0.38 | -0.61 | 4 to 1 | 12.75 | 30.8 | 97.5 | |
| 5 | -0.54 | -0.87 | 3 to 1 | 12.09 | 29.25 | 103 | |
| 4 | -0.85 | -1.36 | 2 to 1 | 10.2 | 24.61 | 122 | |
| 3 | -1.51 | -2.42 | ∞ to 2 | 3.40 | 8.21 | 366 | |
| 2 | -5.42 | -3.40 | 5 to 2 | 2.86 | 6.90 | 435 | |
| 1 | -13.60 | -21.8 | 4 to 2 | 2.55 | 6.15 | 488 | |
| 3 to 2 | 1.89 | 4.56 | 658 | ||||
| ∞ to 3 | 1.51 | 3.64 | 823 | ||||
| 5 to 3 | 0.97 | 2.34 | 1281 | ||||
| 4 to 3 | 0.66 | 1.59 | 1884 | ||||
| 6 to 4 | 0.47 | 1.13 | 2644 | ||||
| 5 to 4 | 0.31 | 74.8 | 4010 | ||||
| 6 to 5 | 0.16 | 0.386 | 7769 |
You should be able to see from the above table that the only lines in the hydrogen spectrum visible to the human eye are those due to transistions from level 5 to level 2, from level 4 to level 2 and from level 3 to level 2 (wavelengths of 435 nm, 488 nm and 658 nm respectively)