

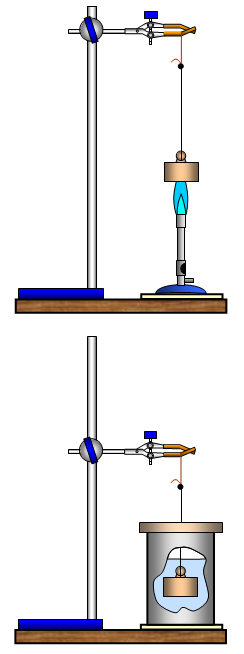
A lump of brass (an old brass balance weight is ideal), an aluminium can (calorimeter) with a cardboard lid to stop water splashing out, a bunsen and protective mat, a piece of string tied to a piece of wire, a pair of tongs, a 0-100oC thermometer, water and a retort stand, boss and clamp
Tie your brass weight firmly to a piece of wire – at least 30 cm long
Measure the mass of the brass weight (mB), the mass of the water in the calorimeter (mW) and the initial temperature of the water in the calorimeter (θo).
Suspend your brass weight from the clamp so that it hangs in the Bunsen flame and heat it strongly for at least two minutes.

| SAFETY CONSIDERATIONS: Use safety goggles here. |
Remove the bunsen and lower the brass rapidly, but carefully, into the water in the calorimeter and replace the lid.
Record the temperature of the water every ten seconds for a minute after you put the weight in, and hence determine the maximum temperature (θF) of the water-metal mixture.
Repeat the experiment twice using different brass weights.
The heat lost by the brass is equal to the heat gained by the water in the calorimeter.
Use your results to determine the initial temperature of the brass. This may be taken as approximately the temperature of the bunsen flame.
Record any sources of error which you consider will affect your result and suggest how they might be reduced.
Was it necessary to record the mass of the calorimeter?

| SAFETY CONSIDERATIONS: Always use the tongs when handling the hot metal objects. |