
Matter can exist is three states, as a solid, a liquid or a gas. Just looking at water in its three states can give us a clue to the molecular structure. The solid form, ice is hard and rigid, a piece of ice has a shape of its own. The liquid form, water is "runny", has no shape of its own and takes up the shape of its container (except in conditions of zero gravity!). The density of liquid water is just slightly higher than that of ice (both at 0oC). Steam is what we think of as water in its gaseous form, this has a very much larger volume (1600 times) than the water that produced it, a low density and no shape of its own. (NB Steam as we see it is not a true gas, the gas produced when water boils is invisible, steam is a cloud of fine droplets of water vapour). The diagrams below show, in a simplified form, how molecules are arranged in a solid, liquid and a gas.
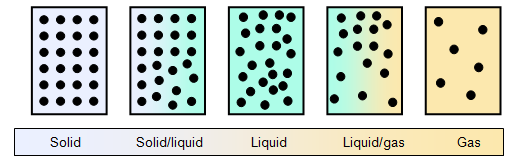
In
a solid the molecules are held together by intermolecular forces, the exact type of force
depending on the type of solid.
These forces are divide into four types:
(a) ionic bonds
- sodium and chloride ions in sodium chloride
(b) covalent bonds - shared electrons
between atoms
(c) metallic bonds - free electrons wandering through a metal
(d) Van
der Waal's bonds - electric dipole forces
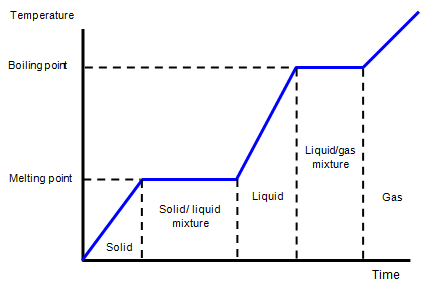
When heat is added to a solid the molecules gain energy and the
temperature rises. As more and more heat is added the molecular vibrations become more
violent and eventually the bonds between the molecules break and the structure changes - this
is melting.
Notice that while the solid is changing to a liquid there
is no rise in temperature. The energy input goes simply to breaking the intermolecular bonds.
Adding still more energy makes the molecules move even faster and then move further apart
as the liquid changes to a gas - the liquid is boiling. Once again during boiling as with melting
there is no change of temperature. This time the energy goes to separating the molecules and
so giving them more potential energy and also to pushing back the surrounding atmosphere.
In a gas the molecules have been separated so much that there is virtually no
attractive force between them.
The energy required to change the state of 1 kg of a
series of substances is shown in the following table.
| Substance | Melting (kJkg-1) | Boiling (MJkg-1) |
| (solid - liquid) | (liquid - gas) | |
| Helium | 5.23 | 0.021 |
| Oxygen | 13.8 | 0.231 |
| Ethanol | 104 | 0.854 |
| Mercury | 1.8 | 0.29 |
| Water | 334 | 2.26 |
| Copper | 210 | 5.07 |