
The ancients such as the philosophers Democritus and
Lucretius held that matter was composed of minute particles. They also Maintained that
these particles were in a state of continuous random motion within solids, liquids and gases.
The theory was therefore called the kinetic theory of matter, after the Greek work kinema -
motion.
It was not until 1827, however, that actual experimental evidence for these
particles existed. This was provided by the Scottish physicist Robert Brown. He observed a
weak solution of milk and later pollen grains in suspension with a high-powered microscope,
and saw that the particles of milk and the pollen grains showed a violent and random motion.
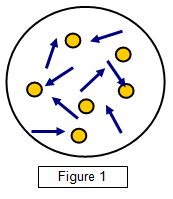
(Figure 1) Brown wrongly attributed what he saw to living organisms, and the true
explanation was not given until some thirty years later when the Frenchman Carbonelle
proposed that the motion was due to the impacts of the liquid molecules on the milk particles
or pollen grains. The motion is now known as Brownian movement.
However the first good explanation of Brownian
movement was advanced by the French scientist Desaulx in 1877: "In my way of thinking the
phenomenon is a result of thermal molecular motion in the liquid environment (of the
particles)."
A simple modern version of Brown's experiment is the smoke cell. A
small cell of air is placed under a microscope and illuminated strongly from the side. Some
smoke is then blown into it. Through the microscope the particles of smoke can be seen to
be in violent random motion just like Brown's pollen grains. This motion is due to the
collisions of the (invisible) air molecules with the much larger particles of smoke. Heating the
cell makes the smoke particles' motion even more violent due to the increased velocity of the
air molecules.

A model of the kinetic theory of gases is shown in the photograph. Small polystyrene beads are held in a plastic tube and vibrated from beneath. The greater the frequency and amplitude of the vibrations the more violent is the motion of the beads. Placing a small polystyrene ball in the cylinder simulates Brownian motion. The beads collide with the ball which then moves randomly just like the motion of the smoke particles in the smoke cell.