Derivation of the kinetic theory
formula
Remember that what follows applies to ideal gases only; the
assumptions that we make certainly do not all apply to solids and liquids.
This proof
was originally proposed by Maxwell in 1860. He considered a gas to be a collection of
molecules and made the following assumptions about these molecules:
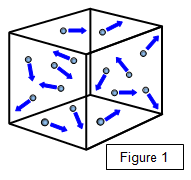
- molecules behave as if they were hard,
smooth, elastic spheres
- molecules are in continuous random motion
- the average
kinetic energy of the molecules is proportional to the absolute temperature of the gas
- the molecules do not exert any appreciable attraction on each other
- the volume of
the molecules is infinitesimal when compared with the volume of the gas
- the time spent
in collisions is small compared with the time between
collisions
Consider a volume of gas V enclosed by a cubical
box of sides L. Let the box contain N molecules of gas each of mass m, and let the density of
the gas be r. Let the velocities of the molecules be u1,
u2, u3 . . . uN. (Figure 2)
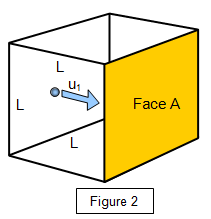
Consider a molecule moving in the x-direction towards face A with
velocity u
1. On collision with face A the molecule will experience a change of momentum
equal to 2mu
1. (Figure 3)
It will then travel back across the box, collide with the
opposite face and hit face A again after a time t, where t = 2L/u
1.
The
number of impacts per second on face A will therefore be 1/t =
u
1/2L.
Therefore rate of change of momentum = [mu
12]/L =
force on face A due to one molecule.
But the area of face A = L
2, so
pressure on face A = [mu
12]/L
3But there are N molecules in the box
and if they were all travelling along the x-direction then
Total pressure on face A =
[m/L
3](u
12 + u
22 +...+ u
N2)
But on
average only one-third of the molecules will be travelling along the x-direction.
Therefore: pressure = 1/3 [m/L
3](u
12 +
u
22 +...+ u
N2)
If we rewrite Nc
2 =
[u
12 + u
22 + …+ u
N2 ] where c is the mean square velocity of
the molecules:
pressure = 1/3 [m/L
3]Nc
2 But
L
3 is the volume of the gas and therefore:
Pressure (P) = 1/3 [m/V]Nc2 and so PV = 1/3 [mNc2]
and this is the kinetic theory
equation.
Now the total mass of the gas M = mN, and since
r = M/V we can write
Pressure (P) = 1/3 [ρc2]
The root mean square velocity or r.m.s. velocity is
written as c
r.m.s. and is given by the equation:
r.m.s. velocity = c
r.m.s.
= [c
2]
1/2 = [u
12 + u
22 + …+ u
N2
]
1/2/N
We can use this equation to calculate the root mean square velocity of gas
molecules at any given temperature and pressure.
Example problem
The density of nitrogen at s.t.p. = 1.251 kg m-3. Calculate the r.m.s. velocity of nitrogen molecules.
c2= 3p/ρ = [3x9.81x13600x0.76]/1.251 = 2.432x105
Therefore: cr.m.s = 493 ms-1
Some further values of the
root mean square velocity at s.t.p. for other gases are given below.
| Gas |
r.m.s. velocity (ms-1 |
| Hydrogen |
18.39x102 |
| Helium |
13.10x102 |
| Oxygen |
4.61x102 |
| Carbon dioxide |
3.92x102 |
| Bromine |
2.06x102 |