
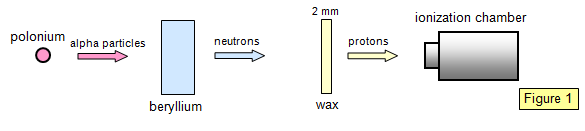
In 1932 Bother and Becker bombarded a
piece of beryllium with alpha particles using the apparatus shown diagrammatically in
Figure 1. They observed a penetrating radiation emerging from the beryllium that they
thought to be gamma rays. When a piece of paraffin wax was placed in the path of the
beam, however, the reading on the detector actually increased!
It was realised that the increase in reading was due
to the emission of protons from the wax and they then tried to work out how this could be
happening. If the proton emission had been due to bombardment of the wax by gamma
radiation the gamma rays would have to have energy of around 16 MeV – very large for
such a reaction.
The British physicist James Chadwick realized that the
radiation produced by the bombardment of the wax by alpha particles was not gamma
radiation at all but particles of about the same mass as the proton. The high penetration
was explained by assuming that these particles were uncharged and could therefore
pass though material without any electrostatic scattering.
The particle was
named the neutron, a name suggested years before
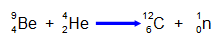
by Rutherford as a combination of a proton and an electron. The reaction between alpha
particles and beryllium that produces neutrons is:
It is for this reason that irradiation
by neutrons is particularly dangerous to human tissue which contains large numbers of
hydrogenous atoms with a mass similar to that of the neutron.
The neutron is
now known to have a mass slightly greater than that of a proton, the neutron's mass
being 1.008 665 u compared with the protons 1.007 276 u. Free neutrons are unstable
decaying into a proton and electron, the process having a half life of about 900 s (15
minutes).
The most common large-scale source of neutrons is the nuclear
fission reactor. Materials are often placed in reactors to see how they stand up to
neutron bombardment and also to create new isotopes by neutron absorption.